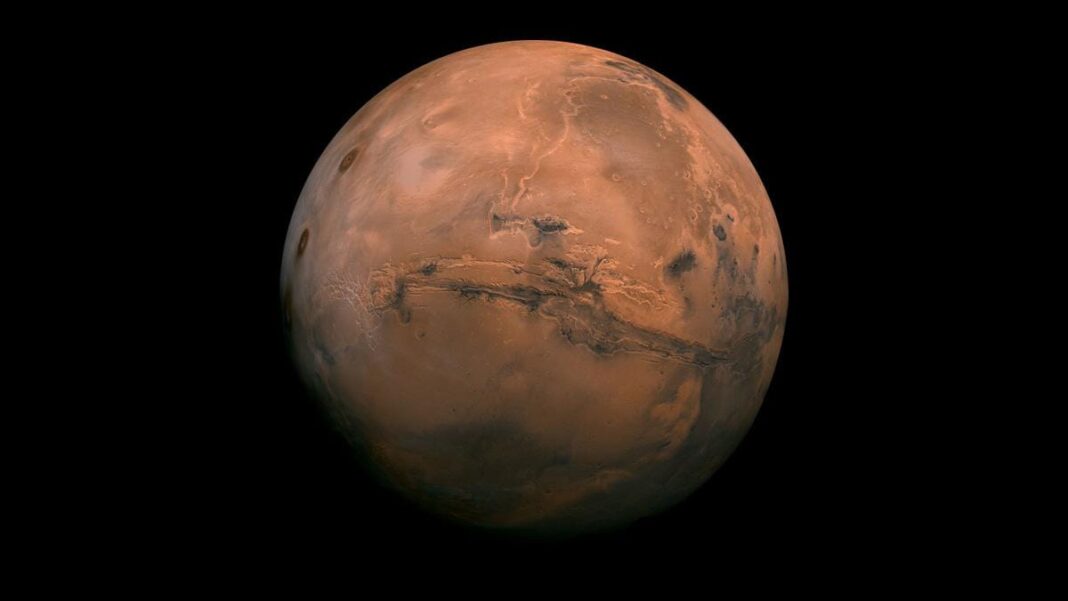
A US team led by an Indian-origin scientist has developed a new system that extracts oxygen from Mars’ salty water.
A new system that can extract oxygen and hydrogen fuel from the salty water on Mars has been developed by a team in the US, led by an Indian-origin scientist.
According to a report in Edexlive, the new finding by the team, led by Vijay Ramani, a professor at the Washington University in the US, has the potential to change the logistics of future mission to Mars and beyond.
How does the new system work?
As per the report, the researchers noted that Mars is very cold, and water that is not frozen is almost certainly full of salt from the Martian soil, which lowers its freezing temperature. Using the existing method of electricity to break the briny water down into oxygen and hydrogen fuel requires removing the salt, which is a cumbersome and a costly endeavour in a harsh, dangerous martian environment, they said.
The team examined the new system in a simulated Martian atmosphere at minus 36 degrees Celsius. “Our Martian brine electrolyser radically changes the logistical calculus of missions to Mars and beyond. This technology is equally useful on Earth where it opens up the oceans as a viable oxygen and fuel source,” the report quoted Ramani as saying. In 2008, Nasa’s Phoenix Mars Lander “touched and tasted” Martian water, vapours from melted ice dug up by the lander.
Oxygen, fuel needed to live on Mars
Since then, the European Space Agency’s Mars Express has discovered several underground ponds of water which remain in a liquid state thanks to the presence of magnesium perchlorate salt. In the journal Proceedings of the National Academy of Sciences (PNAS), the researchers noted that in order to live – even temporarily – on Mars, not to mention to return to Earth, astronauts will need to manufacture some of the necessities, including water and fuel, on the Red Planet, the report said.
New system better than Nasa’s
Nasa’s Perseverance rover that is en-route to Mars will be producing oxygen only, from the carbon dioxide in the air. It will be using the Mars Oxygen In-Situ Resource Utilization Experiment (MOXIE). The system developed in Ramani’s lab can produce 25 times more oxygen than Nasa’s rover using the same amount of power, said the researchers, adding it also produces hydrogen, which could be used to fuel astronauts’ trip home.
“Our novel brine electrolyser incorporates a lead ruthenate pyrochlore anode developed by our team in conjunction with a platinum on carbon cathode” Ramani said. “These carefully designed components coupled with the optimal use of traditional electrochemical engineering principles has yielded this high performance,” the report quoted Ramani as saying. The careful design and unique anode allow the system to function without the need for heating or purifying the water source, the researchers said.
“Paradoxically, the dissolved perchlorate in the water, so-called impurities, actually help in an environment like that of Mars,” Edexlive quoted Shrihari Sankarasubramanian, a research scientist in Ramani’s group as saying. “They prevent the water from freezing and also improve the performance of the electrolyser system by lowering the electrical resistance,” said Sankarasubramanian who is also the joint first author of the research paper on the study.
New system better than water electrolysers
Water electrolysers typically use highly purified, deionized water, which adds to the cost of the system, according to the researchers. A system that can work with “sub-optimal” or salty water, such as the technology demonstrated by the team, can significantly enhance the economic value proposition of water electrolysers everywhere, even on the Earth, they said.
“Having demonstrated these electrolysers under demanding Martian conditions, we intend to also deploy them under much milder conditions on Earth to utilize brackish or salt water feeds to produce hydrogen and oxygen, for example through seawater electrolysis,” the report quoted Pralay Gayen, a postdoctoral research associate in Ramani’s group as saying. He is also a joint first author on the study.
Such applications could be useful in the defence realm, creating oxygen on demand in submarines, for example, said the researchers, adding it could also provide oxygen as we explore uncharted environments in the deep sea.












![Hotstar Premium Cookies 2019 [*100% Working & Daily Updated*] Hotstar Premium Cookies 2019 [*100% Working & Daily Updated*]](https://tahav.com/wp-content/uploads/2019/11/Hotstar-Premium-Cookies-Free-100x70.jpg)



